Coordination Engineering Modulates Spin-Polarization in Ruthenium Oxide to Enhance Acidic Oxygen Evolution Reaction
Abstract
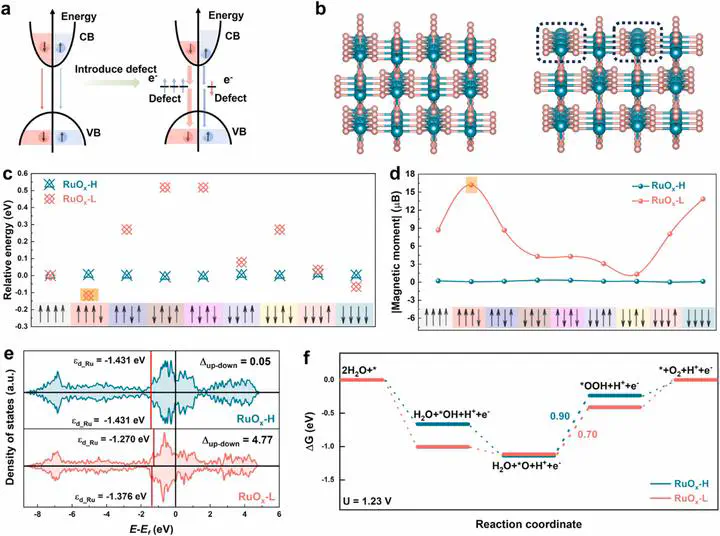
ABSTRACT Spin catalysis, which involves the enhancement of electrocatalytic reactions by mitigating the constraints on electron angular momentum interactions between reactants (e.g., diamagnetic OH⁻/H2O) and products (e.g., triplet-state O2), holds significant potential to transform the field of oxygen evolution reactions (OER). Despite this promise, strategies for controlling spin-polarization and a thorough understanding of the underlying spin catalysis mechanism remain considerable challenges. Here, we present a coordination engineering strategy to enhance the intrinsic OER activity of non-ferromagnetic rutile ruthenium oxide (RuO2) through spontaneous spin-polarization. Based on density functional theory (DFT) calculations and experimental characterizations, we demonstrate that moderate interface unsaturation in the coordination environment induces a reconfiguration of the electron spin structure at the interface, reducing the energy barrier for OER. The optimized RuOx with low coordinated configuration (RuOx-L) exhibits enhanced intrinsic catalytic activity, with a low overpotential of 217mV at 10mAcm-2 and stable performance over 160hours. When integrated into proton exchange membrane water electrolysis, RuOx-L delivers 1.0Acm-2 at 1.79V, maintaining stable performance for 100hours. This work introduces a facial strategy for inducing spontaneous spin-polarization and offers new insights into the intrinsic mechanisms of spin electrocatalysis.
Ruthenium dioxide (RuO₂) is a leading catalyst for the acidic oxygen evolution reaction (OER), yet its performance is limited by high overpotential and long-term stability issues. This study introduces a simple “coordination engineering” strategy to fine-tune the local Ru–O environment and thus regulate Ru’s spin states, achieving substantial improvements in OER activity and durability.
Key Strategies
Vacancy Creation (RuO₂–V): Controlled reduction–oxidation cycles generate oxygen vacancies, decreasing the Ru–O coordination number from 6 to ≈5.5.
Heteroatom Doping (RuO₂–M): Incorporation of trace Mn or Fe slightly increases coordination to ≈6.3.
Major Findings
Enhanced Spin-PolarizationVacancy and doping treatments boost the unpaired electron count at Ru sites, shown by ESR and magnetometry: magnetic moments rise from ~0.15 μ_B (pristine) to 0.50 μ_B.
Lower Overpotentials
RuO₂–V: 250 mV at 10 mA cm⁻² (−60 mV vs. pristine)
RuO₂–Mn: 235 mV (best performance)
Improved Kinetics and StabilityTafel slopes drop from 85 mV dec⁻¹ to 55–60 mV dec⁻¹, and 10 h stability tests retain >95% current.

Theoretical InsightsDFT calculations reveal that optimized spin splitting lowers the free energy for the *OOH intermediate (1.25 eV → 0.95 eV), cutting theoretical overpotential to ≈0.1 V. Enhanced overlap of Ru 4d↑ and O 2p↓ orbitals promotes spin-selective electron transfer.
Implications This work pioneers using spin-polarization as a design parameter in OER catalysis. By establishing a clear link between coordination number, magnetic moment, and catalytic performance, it offers a transferable guideline for other metal oxides (IrO₂, Co₃O₄, etc.). Future directions include in situ spin-state tracking (XMCD) and validation in full PEM electrolyzers.