Atomic Ga triggers spatiotemporal coordination of oxygen radicals for efficient water oxidation on crystalline RuO2
Abstract
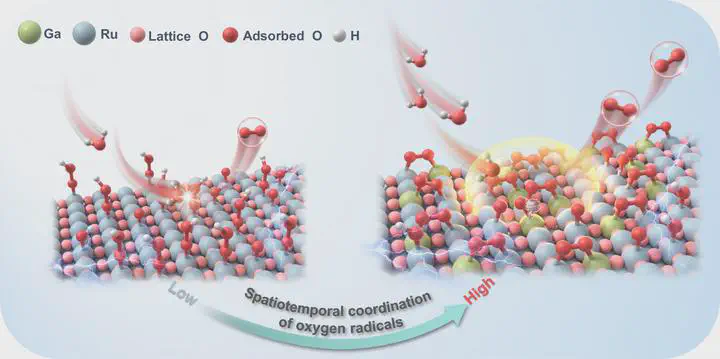
Advancements in proton-exchange membrane water electrolyzer depend on developing oxygen evolution reaction electrocatalysts that synergize high activity with stability. Here, we introduce an approach aimed at elevating oxygen evolution reaction performance by enhancing the spatiotemporal coordination of oxygen radicals to promote efficient O-O coupling. A dense, single-atom configuration of oxygen radical donors within interconnected RuO2 nanocrystal framework is demonstrated. The stable oxygen radicals on gallium sites with adaptable Ga-O bonds are thermodynamically favorable to attract those from Ru sites, addressing dynamic adaptation challenges and boosting O-O coupling efficiency. The optimized catalyst achieves a low overpotential of 188 mV at 10 mA cm-2, operates robustly for 800 h at 100 mA cm-2 in acidic conditions, and shows a large current density of 3 A cm-2 at 1.788 V, with stable performance at 0.5 A cm-2 for 200 h, confirming its long-term viability in proton-exchange membrane water electrolyzer applications.